The University Record, April 8, 1998
By Sally Pobojewski
Health System Public Relations
|
|
|
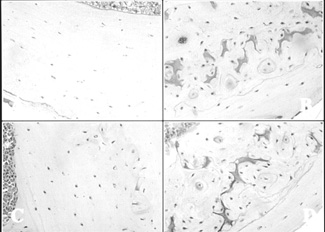
Bone sections from (A) 16-week-old littermate control mice, (B) 16-week-old transgenic mice, (C) 30-week-old littermate control mice, and (D) 30-week-old transgenic mice (Magnification x100). Transgenic sections show darkly-stained calcified cartilage, irregular-patterned woven bone and surface irregularities. Reproduced from J Bone Miner Res 1998; 13:706-715 with permission of the American Society for Bone and Mineral Research. |
Laboratory mice that have been genetically altered to produce human growth hormone grow to be 25-30 percent larger than normal mice–with much of that size difference coming from bigger bones, according to U-M researchers. The trade-off for bigger bones in adolescence, however, appears to be poorer bone quality, increased brittleness and less resistance to fractures as mice age.
Steven A. Goldstein, professor of surgery and of biomedical engineering, suggests his results, while preliminary, raise a “red flag” on the increasing use of human growth hormone in short children and as a “fountain-of-youth drug” in the elderly.
The study by Kuo-Fung Tseng, now with the Veterans General Hospital in Taipei, Taiwan, and Goldstein is published in the April 1998 issue of the Journal of Bone and Mineral Research. The study was designed to learn more about complex feedback mechanisms between genes and mechanical forces, which control how bones grow and develop. “This balance between structure and function has evolved over years of mammalian evolutionary development to produce bone strong enough for an animal’s daily activity, but not so strong that it requires too much energy to grow and maintain,” Goldstein says.
Thirty-nine of the male mice in the study were transgenic–meaning that their DNA contained both mouse and human genes. These mice were able to secrete large amounts of human growth factor, because the human gene that triggers production of this hormone had been inserted into their DNA during the fertilized-egg stage of development. Thirty-six male control mice in the study were littermates of the experimental mice, but did not carry the gene for human growth hormone.
According to Goldstein, mean body weights of the two groups were identical until the puberty growth spurt, which begins when mice are between four and nine weeks old. “After 12 weeks of age, the transgenic mice were 25 to 30 percent larger in body weight and size than their littermate controls,” Goldstein says. “At 16 weeks, the femurs or long upper leg bones of the experimental mice were significantly larger, thicker and longer than those of the control mice.”
“We saw a continual degradation of mechanical properties in the bones of transgenic mice as they aged,” Goldstein says. When they examined cross-sections of bone under a microscope, Tseng and Goldstein found the transgenic mice had more cartilage, woven bone and porous areas in their bone tissue and a lower ash content than did the same bones in age-matched control mice. They also found that transgenic mice had higher levels of bone resorption as they aged than did normal mice.
Goldstein says more research will be necessary to pin down exactly how growth hormone upsets the natural balance between structure and function in developing mouse bone.
“Until we know more, I would be cautious about prescribing human growth hormone, especially to individuals without a specific medical need, without giving any thought to the possible consequences,” Goldstein says.
The study was funded by the National Institutes of Health and the Frederick Fischer Endowment for pediatric orthopedic research at the U-M. Jeffrey F. Bonadio, a Medical School senior associate research scientist, also was a key collaborator in the research.