The University Record, September 30, 1998
New anti-microbial agent destroys anthrax, kills flu virus
By Sally Pobojewski
Health System Public Relations
 BCTP looks like skim milk. Laboratory rats gain weight when they eat it. Spray it on your lawn and the grass will thrive. But according to tests conducted by U-M scientists, this seemingly benign material could be a potent weapon against anthrax–one of the deadliest bacteria on Earth. It also has been found to be a quick and efficient killer of influenza A virus in cell cultures and in the nasal passages of laboratory mice.
BCTP looks like skim milk. Laboratory rats gain weight when they eat it. Spray it on your lawn and the grass will thrive. But according to tests conducted by U-M scientists, this seemingly benign material could be a potent weapon against anthrax–one of the deadliest bacteria on Earth. It also has been found to be a quick and efficient killer of influenza A virus in cell cultures and in the nasal passages of laboratory mice.
BCTP destroys anthrax, but doesn’t hurt animals or the environment
In a presentation at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) on Sept. 26, Medical School research associate Michael Hayes presented experimental evidence of BCTP’s ability to destroy anthrax spores both in a culture dish and in mice exposed to anthrax through a skin incision. James R. Baker Jr., professor of internal medicine and director of the Center for Biologic Nanotechnology, directed the research study.
BCTP was developed by D. Craig Wright, chief research scientist at Novavax Inc.–a bio-pharmaceutical company in Columbia, Md.–and president of Novavax Biologics Division. The material is made of water, soybean oil, Triton X 100 detergent and the solvent tri-n-butyl phosphate.
“One of the most remarkable characteristics of this material is its ability to rapidly destroy a wide variety of dangerous bacteria and viruses, while remaining non-toxic to people, animals or the environment,” Baker says.
BCTP’s effectiveness against anthrax spores is especially significant because they are so difficult to kill. “Spores are like freeze-dried bacteria,” Baker explains. “Their tough outer coat is resistant to disinfectants, freezing, drought, virtually anything we can throw at them. Spores can survive in the environment for many years and still generate live bacteria when given the right combination of water, nutrients and temperature.”
Concentrated doses of strong disinfectants like bleach or formaldehyde will kill anthrax spores, according to Baker. Unfortunately, they also are toxic to people and the environment, which makes them useless for decontaminating a person, a piece of land or equipment exposed to the bacteria.
Since the Persian Gulf War, military authorities have become increasingly concerned about the threat that anthrax and other biological warfare agents pose both to our armed forces and civilian populations.
“Anthrax is often fatal and easily dispersed through air or water,” Baker says. “We know that countries hostile to the United States have developed strains of anthrax which are resistant to antibiotics and existing vaccines. To counter that threat, the Defense Advanced Research Projects Agency (DARPA) is testing several possible new weapons against these biologic agents–including BCTP.”
“When properly formulated, the components in BCTP form an emulsion of tiny lipid droplets suspended in solvent,” Wright says. “These lipids fuse with anthrax spores causing the spore to revert to its active bacterial state. During this process, which takes four to five hours, the spore’s tough outer membrane changes, allowing BCTP’s solvent to strip away the exterior membrane. BCTP’s detergent then degrades the spore’s interior contents. In scanning electron microscope images, the spores appear to explode.”
In his conference presentation, Hayes described how even low concentrations of BCTP killed more than 90 percent of virulent strains of Bacillus anthracis spores in a culture dish. “We observed sporicidal activity with dilutions as high as one part BCTP per 1,000 parts culture media,” Hayes said.
To determine its toxicity to animals, U-M scientists fed large amounts of BCTP to laboratory rats and injected mice with the material subcutaneously. The animals gained weight, remained healthy and suffered no adverse effects.
To determine BCTP’s effectiveness at treating animals exposed to anthrax spores, Baker’s research team injected mice subcutaneously with Bacillus cereus–a closely related species of bacteria that can be safely handled in a university laboratory setting. Like B. anthracis, its lethal relative, B. cereus produces large, ulcerous areas of dead tissue if it penetrates the skin through a cut or injury. If untreated, these skin infections spread systemically, producing severe illness and death in 80 percent of the laboratory mice in the study.
“When we washed the animal’s skin lesions with BCTP, the wounds began to heal,” Baker said. Mice receiving BCTP either simultaneously with B. cereus spores or whose wounds were washed with BCTP an hour after exposure had a 95 percent reduction in lesion size. The death rate for mice receiving BCTP was only 20 percent.
“Rapid inactivation of anthrax bacteria and spores combined with low toxicity makes BCTP a promising candidate for use as a broad-spectrum, post-exposure decontamination agent,” Baker says.
In future studies, Baker plans to evaluate BCTP’s effectiveness against inhaled anthrax spores, as well as other bacteria and enveloped viruses.
BCTP kills influenza virus and prevents infection in mice
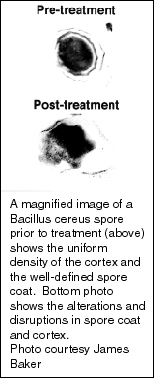 The research studies on BCTP and the influenza virus “are preliminary and small-scale, but the results indicate that BCTP shows promise as a new weapon against the influenza A virus,” Baker says. “Its main advantages are its rapid killing action, lack of specificity and the fact that it is non-toxic to skin and mucous membranes.”
The research studies on BCTP and the influenza virus “are preliminary and small-scale, but the results indicate that BCTP shows promise as a new weapon against the influenza A virus,” Baker says. “Its main advantages are its rapid killing action, lack of specificity and the fact that it is non-toxic to skin and mucous membranes.”
U-M research associates Andrzej Myc (internal medicine-allergy) and Jon D. Reuter (Unit for Laboratory Animal Medicine) presented results of preliminary studies evaluating BCTP’s effect on influenza A at the Interscience Conference. The studies were funded by the Defense Advanced Research Projects Agency and directed by Baker.
Myc’s study used Madin Darby Canine Kidney (MDCK) cells, used by researchers to evaluate the toxic effects of viruses. Myc incubated MDCK cells with influenza A virus and five different formulations of Novavax lipid structures. Using two different assay techniques, Myc then measured the number of cells infected with the virus. While all five formulations slowed the spread of the virus, BCTP was the most potent, reducing viral antigen levels by 99.6 percent.
In Reuter’s study, different liquids were inserted into the nasal passages of four groups of laboratory mice. Control mice in Group 1 were given ordinary saltwater. Group 2 received BCTP alone. Group 3 received live influenza A virus and Group 4 was given a mixture of influenza A and BCTP. Groups 1, 2 and 4 stayed healthy, while all the mice in Group 3 developed severe pneumonia and two out of three mice died before the conclusion of the study.
“We learned several important things from these preliminary studies. The first is that BCTP is a highly effective killing agent for the influenza virus both at the cellular level and in living animals. Equally important is that BCTP had no toxic effects on nasal or lung membranes,” Baker says. “We’ve shown that if we treat the virus with BCTP as it enters the nasal passages, we can prevent infection in mice. The next step is to see whether we can administer BCTP and the virus separately and still prevent infection. And the final step, of course, is to see whether it works as well in people as it does in mice.”
While influenza vaccines are relatively effective at preventing the flu, Baker says there is a need for alternate preventive agents. “Influenza vaccines are expensive, they only are effective against a few viral strains each year and it takes time for immunity to develop. BCTP appears to inactivate the virus on contact.”
Baker’s research is funded by DARPA’s Unconventional Pathogen Countermeasures Program. The U-M and Novavax have filed a patent application covering BCTP’s use as a decontamination agent for various anti-microbial applications. Baker is a member of the Novavax scientific advisory board, but has no significant financial interest in the company.
You can always drop us a line: [email protected].

