By Kara Gavin
Health System Public Relations
Cancer patients who now endure months of treatment—and then weeks of anxious waiting to see whether it worked—may soon get word of their tumors’ response within days of starting therapy, thanks to a new use for a widely available MRI technique.
In the first study of its kind, researchers from the Cancer Center are reporting success in analyzing diffusion MRI images to distinguish between dead and living brain tumor cells in both animals and humans. They report that the imaging technique, which tracks the movement of water through and between cells, allowed them to assess the effect of therapy on the cancer without relying on measurable changes in tumor size.
Published in the Dec. 20 issue of the Journal of the National Cancer Institute, the early finding has the potential to dramatically change the way physicians plan and track the effectiveness of the cancer therapies they prescribe. It could spare patients the physical side effects of weeks of unsuccessful treatment and the psychological effects of then waiting a month or more for an MRI scan to show whether they’re responding. It could aid in testing new anti-cancer agents.
“One of the biggest problems in dealing with many solid cancers is measuring their response to treatment in a timely way,” says corresponding author Brian Ross. “Diffusion MRI seems to provide a way to gauge that response faster and could individualize the clinical management of each patient.” Ross is associate professor of radiological sciences and biological chemistry and co-director, with Alnawaz Rehemtullah, co-author and associate professor of radiation oncology, of the Health System’s Center for Molecular Imaging.
Already widely used to diagnose strokes, diffusion MRI can be done using nearly any closed MRI scanner, and it adds just a few minutes to a regular scan, says lead author and radiology Prof. Thomas Chenevert. The new study looks at brain tumors, but the U-M team has already shown the technique is useful in other solid cancers in animals and humans.
The researchers stress that the technique is not yet ready for use in planning a cancer patient’s treatment. They’re working with colleagues elsewhere on further studies of the approach, continuing to test it at the U-M, and are planning a multi-center clinical trial for late this year.
MRI, which stands for magnetic resonance imaging, makes images of the body’s inner structure using a strong magnetic field that aligns the body’s water molecules. Since water makes up the bulk of every kind of tissue, the images reflect the differences among various tissues’ water content and density.
MRI is one of the most common forms of medical imaging technology and is routinely used before and after cancer treatment to help physicians measure the size of tumors and assess a patient’s progress.
But even though cancer treatments such as chemotherapy or radiation kill tumor cells immediately, it often can be weeks before the body absorbs enough of the dead cells to produce a change in tumor size that’s visible on an MRI scan.
The U-M team’s approach seeks a more rapid answer using the extra information provided by diffusion MRI, which takes the concept of tracking water molecules a step further. Diffusion MRI can assess how easily water is moving across microscopic distances—a continuous process called diffusion. Healthy cells have unbroken outer membranes that slow water’s movement, but the membranes around dying or dead cells break down, allowing water to diffuse freely.
MRI machines can be programmed to be sensitive to the ease of this water movement in different areas of tissue. The use of diffusion MRI in stroke cases, for example, gives doctors a rapid view of brain regions where blood flow is cut off by a blood hemorrhage or clot.
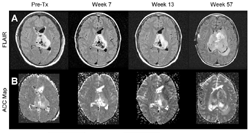
The U-M post-scan processing technique takes the information from a diffusion MRI scan and analyzes it to give a measure of cells’ membrane integrity throughout and near a tumor. It uses a measurement called the apparent diffusion coefficient, or ADC.
The new study compared scans taken before and after the animals and humans were treated for their brain tumors.
The animal study looked at 16 rats with induced tumors treated with a range of doses of the chemotherapy drug BCNU. They had MRIs before treatment and every other day following treatment. Diffusion increased in the first week, even as tumor size grew. On the eighth day, ADC peaked. Soon after, the tumors began to regress, though they later grew back from surviving cells. The magnitude and length of the diffusion response increased with dose.
The two early human patients—a 13-year-old girl and a 37-year-old man—agreed to let the researchers analyze the conventional and diffusion MRI scans they received before, during and after treatment. Both had surgery and chemotherapy preceded or followed by radiation therapy.
The girl’s tumor stopped growing but did not shrink appreciably following initial radiation treatment. It began growing again almost a year later. Early on in treatment, diffusion increased slightly during several months of therapy. Around the time the tumor began growing again, the ADC went down dramatically, corresponding to an increase in the number of intact cell membranes. The man’s tumor responded better. Six weeks after treatment began, the ADC peaked, going up by 86 percent. Over time, his tumor shrank to about one-half its original size.
The research was sponsored in part by the Charles A. Dana Foundation, the National Cancer Institute and the U-M Clinical Research Partnership Fund.

