Common test may overestimate mercury exposure from dental fillings
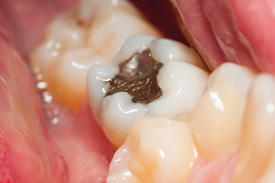
A common test used to determine mercury exposure from dental amalgam fillings may significantly overestimate the amount of the toxic metal released from fillings, according to U-M researchers.
Scientists agree that dental amalgam fillings slowly release mercury vapor into the mouth. But both the amount of mercury released and the question of whether this exposure presents a significant health risk remain controversial.
Public health studies often make the assumption that mercury in urine can be used to estimate exposure to mercury vapor from amalgam fillings. These same studies often use mercury in hair to estimate exposure to organic mercury from a person’s diet.
But a U-M study that measured mercury isotopes in the hair and urine from 12 Michigan dentists found that their urine contained a mix of mercury from two sources: the consumption of fish containing organic mercury and inorganic mercury vapor from the dentists’ own amalgam fillings.
“These results challenge the common assumption that mercury in urine is entirely derived from inhaled mercury vapor,” said Laura Sherman, a postdoctoral research fellow in the Department of Earth and Environmental Sciences and lead author of a paper in the journal Environmental Science & Technology. A final version of the paper was published online March 20.
“These data suggest that in populations that eat fish but lack occupational exposure to mercury vapor, mercury concentrations in urine may overestimate exposure to mercury vapor from dental amalgams. This is an important consideration for studies seeking to determine the health risks of mercury vapor inhalation from dental amalgams,” said U-M biogeochemist Joel D. Blum, a co-author of the paper and a professor in the Department of Earth and Environmental Sciences.
The study by Sherman, Blum and their colleagues demonstrates that mercury isotopes can be used to more accurately assess human exposure to the metal — and the related health risks — than traditional measurements of mercury concentrations in hair and urine samples. Specifically, isotopes provide a novel chemical tracer that can be used to “fingerprint” both organic mercury from fish and inorganic mercury vapor from dental amalgams.
In addition to Sherman and Blum, co-authors of the paper are Alfred Franzblau and Niladri Basu of the School of Public Health.
— Jim Erickson, News Service
Young children endorse sharing but end up hoarding, study shows
Children as young as 3 years old understand they should share with others, but they fail to follow this rule until ages 7 or 8, according to a new U-M study.
“There is abundant evidence that children are aware of fairness standards at a young age, yet young children often allocate resources unfairly when they stand to benefit,” said Craig Smith, a U-M postdoctoral psychology researcher and the study’s lead author.
Smith and colleagues Peter Blake of Boston University and Paul Harris of Harvard University wanted to learn more about the gap between children’s judgment and their behavior. The study also shed light on the youngsters’ willpower when faced with the actual decision of sharing.
The study involved 102 children, whose ages ranged from 3-8. Each received four stickers. With their parents nearby, the children were asked how many stickers they should share with another child. Overall, they thought sharing was the right thing to do in this situation in which both parties were equally deserving, the researchers said.
However, this is where the similarities between the age groups ended. When the moment occurred to share, younger children hoarded their stickers by offering less than an equal split. By 7-8 years of age, children equally shared their treasure.
Perhaps young children fail to share equally because they expect others to hoard, although this doesn’t seem to be the case, the researchers said. “In fact, they expected other children to share at least half of the stickers in the same situation,” Smith said.
The researchers also noted that younger children may have limited self-control regarding fairness when faced with a conflict between sharing and their impulse to take for themselves. However, when asked, surprisingly these younger children correctly predicted that they would share less than half.
— By Jared Wadley, News Service
Paint-on plastic electronics: Aligning polymers for high performance
Semiconducting polymers are an unruly bunch, but U-M engineers have developed a new method for getting them in line that could pave the way for cheaper, greener, “paint-on” plastic electronics.
“This is for the first time a thin-layer, conducting, highly aligned film for high-performance, paintable, directly writeable plastic electronics,” said Jinsang Kim, associate professor of materials science and engineering, chemical engineering, macromolecular science and engineering, and biomedical engineering. Kim led the research published in Nature Materials.
Semiconductors are the key ingredient for computer processors, solar cells and LED displays, but they are expensive. Inorganic semiconductors like silicon require high temperatures in excess of 2,000 degrees Fahrenheit and costly vacuum systems for processing into electronics, but organic and plastic semiconductors can be prepared on a basic lab bench.
The trouble is that charge carriers, like electrons, can’t move through plastics nearly as easily as they can move through inorganic semiconductors, Kim said. Part of the reason for this is because each semiconducting polymer molecule is like a short wire, and these wires are randomly arranged.
“Charge mobility along the polymer chains is much faster than between the polymers,” Kim said.
To take advantage of the good conduction along the polymers, research groups have been trying to align them into a charge-carrying freeway, but it’s a bit like trying to arrange nanoscopic linguine.
Kim’s group approached the problem by making smarter semiconducting polymers. They wanted a liquid polymer solution they could brush over a surface, and the molecules would automatically align with one another in the direction of the stroke, assembling into high-performance semiconducting thin-layer films.
First, they designed the polymers to be slippery — ordinary polymers glom together like flat noodles left in the fridge, Kim said. By choosing polymers with a natural twist, the team kept them from sticking to one another in the solution. But in order to align during the brushstroke, the polymers needed to subtly attract one another. Flat surfaces would do that, so the team designed their polymer to untwist as the solvent dried up.
They stopped the unaligned polymers from forming large chunks by adding flexible arms that extended off to the sides of the flat, wire-like polymer. These arms prevented too much close contact among the polymers while the bulkiness of the arms kept them from snagging on one another. Polymers with these properties will line up in the direction of an applied force, such as the tug of a paintbrush.
“It’s a big breakthrough,” Kim said. “We established a complete molecular design principle of semiconducting polymers with directed alignment capability.”
— Kate McAlpine, College of Engineering
Better than X-rays: A more powerful terahertz imaging system
Low-energy terahertz radiation potentially could enable doctors to see deep into tissues without the damaging effects of X-rays, or allow security guards to identify chemicals in a package without opening it. But it’s been difficult for engineers to make systems powerful enough to accomplish these promising applications.
Now an electrical engineering research team at U-M has developed a laser-powered terahertz source and detector system that transmits with 50 times more power and receives with 30 times more sensitivity than existing technologies. This offers 1,500 times more powerful systems for imaging and sensing applications.
“With our higher-sensitivity terahertz system, you could see deeper into tissues or sense small quantities of illegal drugs and explosives from a farther distance. That’s why it’s important,” said Mona Jarrahi, assistant professor of electrical engineering and computer science, and leader of the project.
Jarrahi’s research team accomplished this by funneling the laser light to specifically selected locations near the device’s electrode that feeds the antenna that transmits and receives the terahertz signal.
Their approach enables light to hitch a ride with free electrons on the surface of the metallic electrodes to form a class of surface waves called surface plasmon waves. By coupling the beam of light with surface plasmon waves, the researchers created a funnel to carry light into nanoscale regions near device electrodes.
The excited surface plasmon waves carry optical photons where they need to be much faster and much more efficiently, Jarrahi said.
The study, “Significant performance enhancement in photoconductive terahertz optoelectronics by incorporating plasmonic contact electrodes,” is published in the current edition of Nature Communications.
In addition to Jarrahi, authors include Christopher Berry and Ning Wang, U-M doctoral students in electrical engineering and computer science; and Mohammad Reza Hashemi and Mehmet Unlu, U-M postdoctoral researchers in electrical engineering and computer science.
— Nicole Casal Moore, News Service

